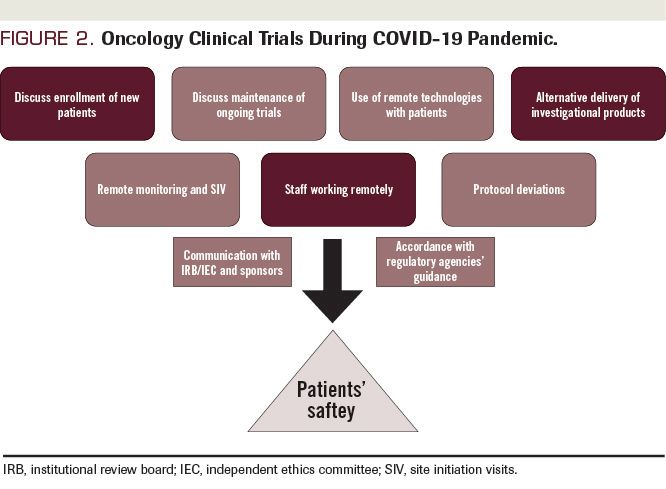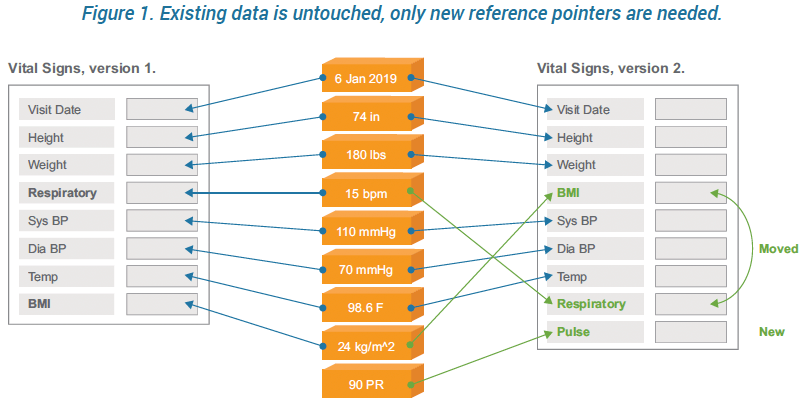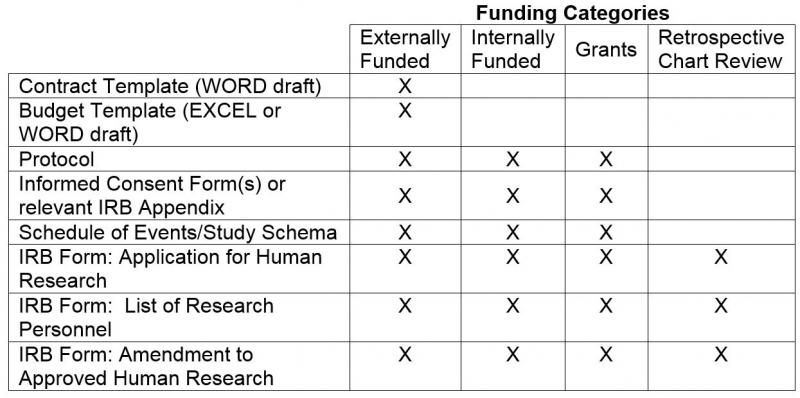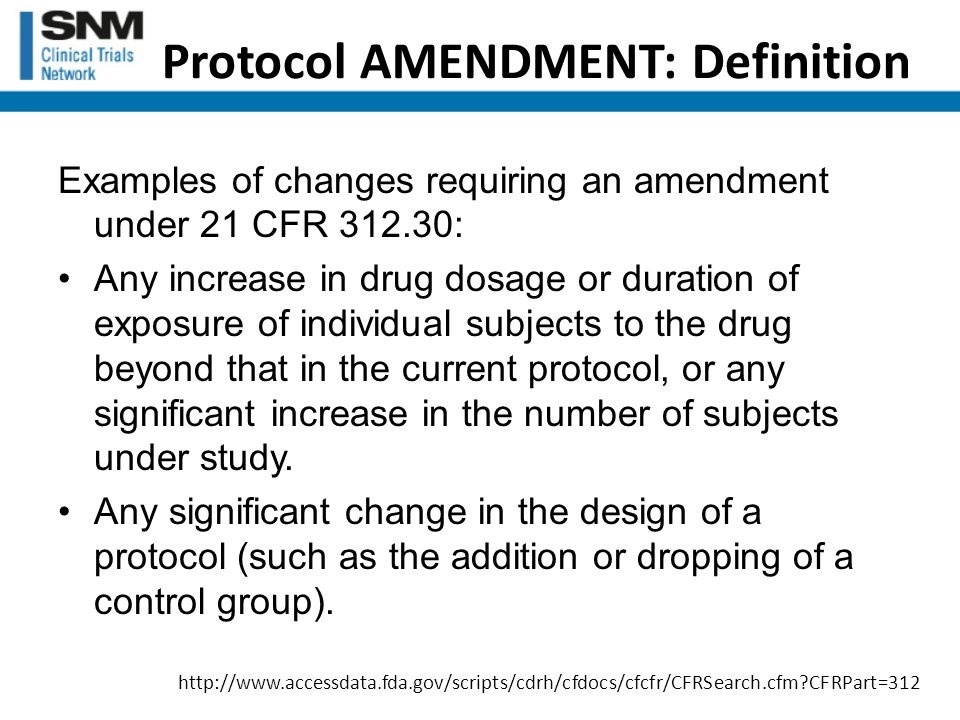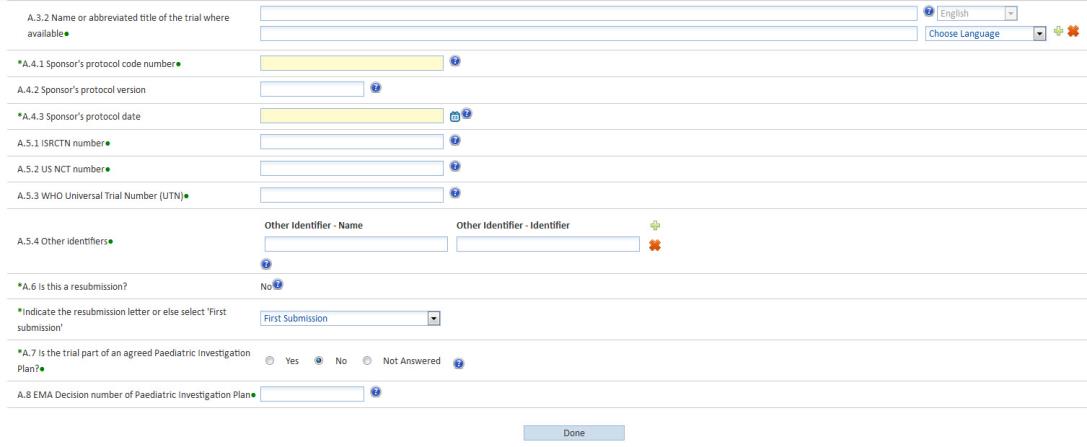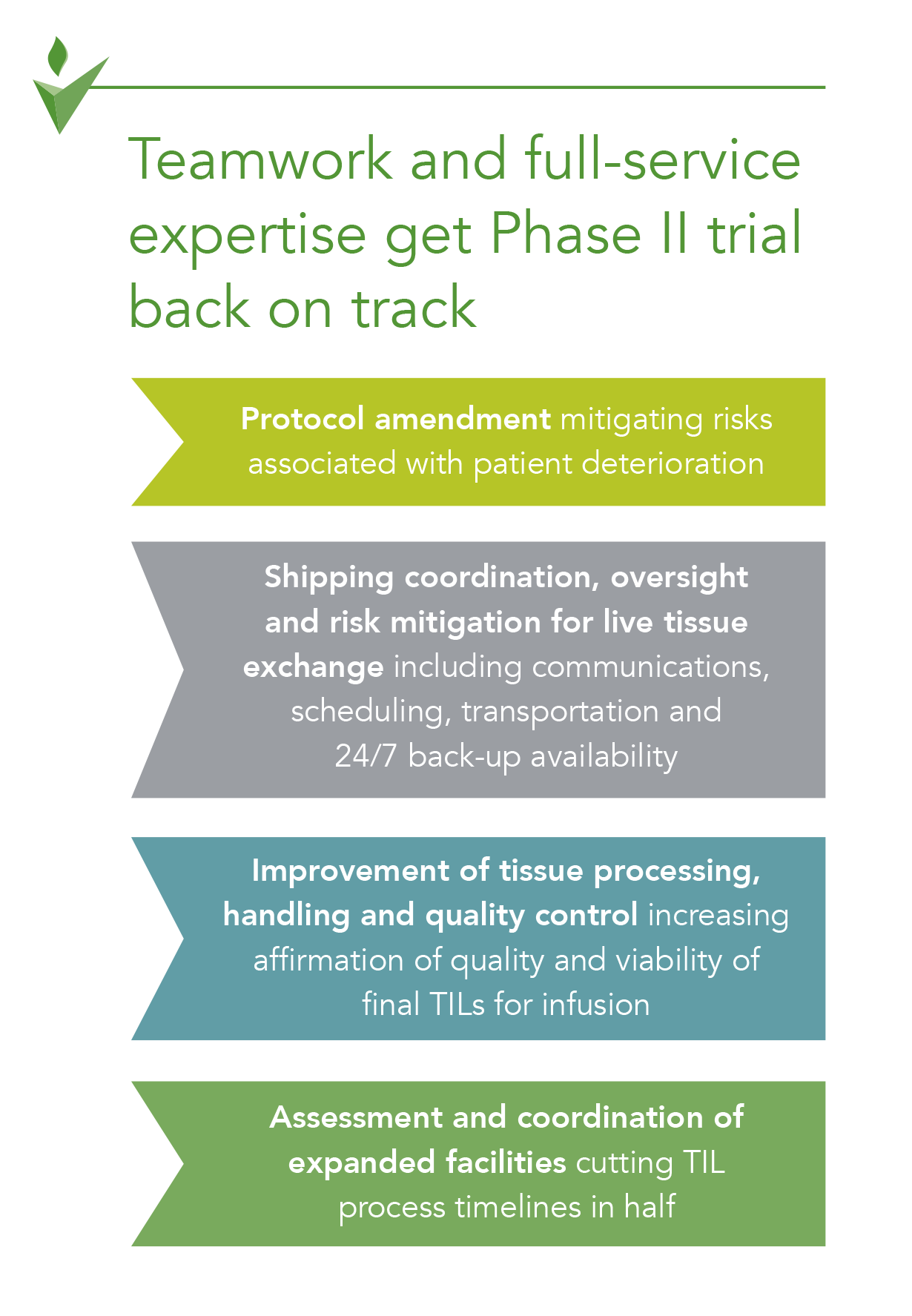
George Clinical Guides Sponsor with Limited Clinical Trial Experience in Phase II Melanoma-TIL Trial – George Clinical

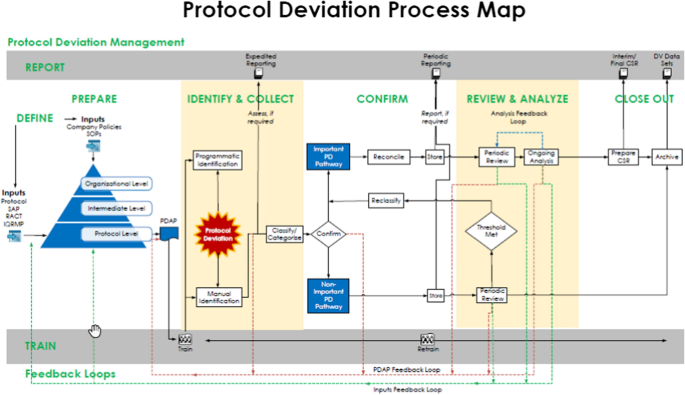
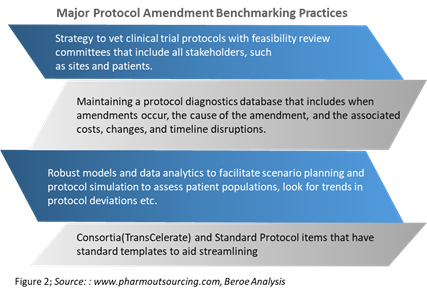
Diagnosing Protocol Amendment Experience to Drive Clinical Trial Performance | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
The statistical analysis of a clinical trial when a protocol amendment changed the inclusion criteria | BMC Medical Research Methodology | Full Text