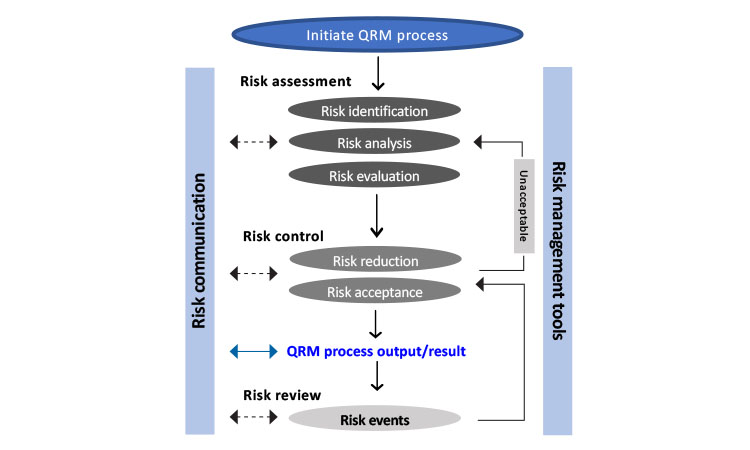
ICH Good Clinical Practice (GCP) E6 (R2) for Investigators and Clinical ... | Clinic, Electronic health records, Clinical research
Creating and testing a GCP game in an asynchronous course environment: The game and future plans | Journal of Clinical and Translational Science | Cambridge Core

good clinical practice.pdf - E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry U.S. Department of Health and Human | Course Hero

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Clinicubes on Twitter: "✔️ICH GCP E6 (R2) FDA Guidance has been released. ❗An important read for all clinical research professionals: ➡️https://t.co/ok3QmHsaWI #ClinicalTrials #GCP #GxP #Trals #FDA #clinicalresearch #ICHGCPE6 https://t.co ...

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

Introduction To Investigators Responsibilities With Good Clinical Practice | PDF | Institutional Review Board | Clinical Trial

ICH GCP Guidelines E6 Revision, R2 Addendum – Changes Impacting Sponsors-CRO-Sites – Compliance Trainings