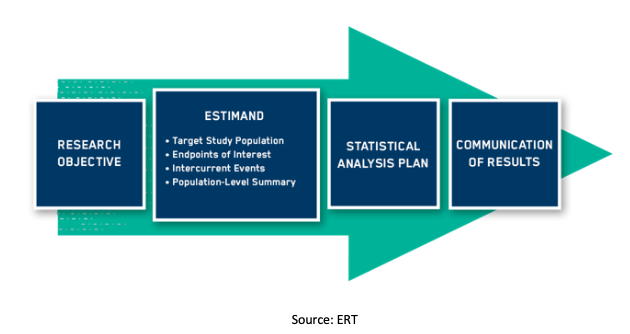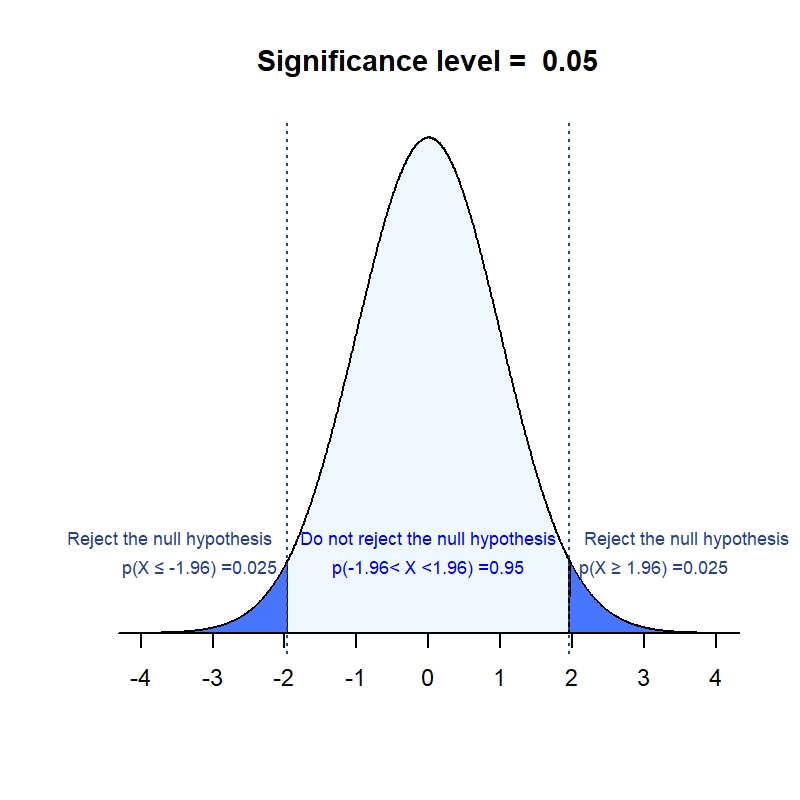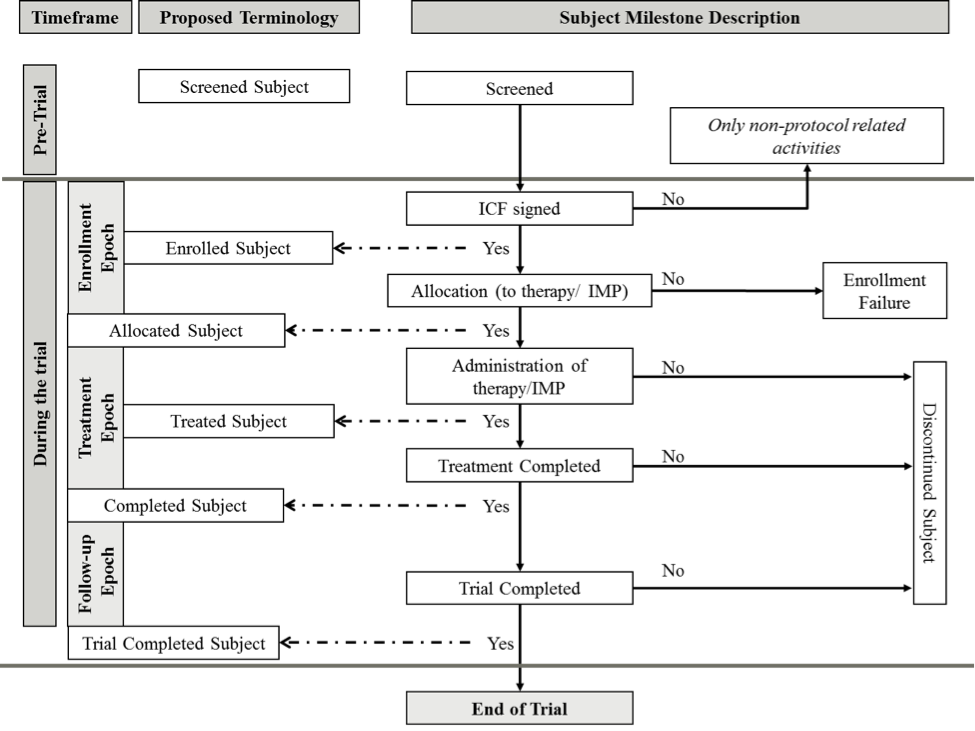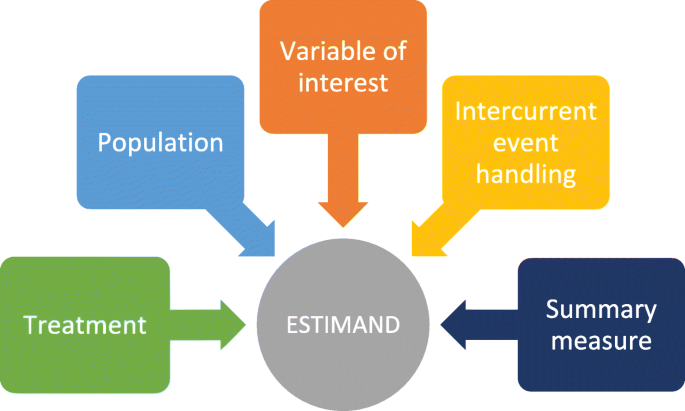
What is an estimand & how does it relate to quantifying the effect of treatment on patient-reported quality of life outcomes in clinical trials? | Journal of Patient-Reported Outcomes | Full Text

Book 13: 2021 FDA Guidance on Clinical Study Reports and Statistical P – Clinical Research Resources, LLC

Early phase clinical trials extension to guidelines for the content of statistical analysis plans | The BMJ
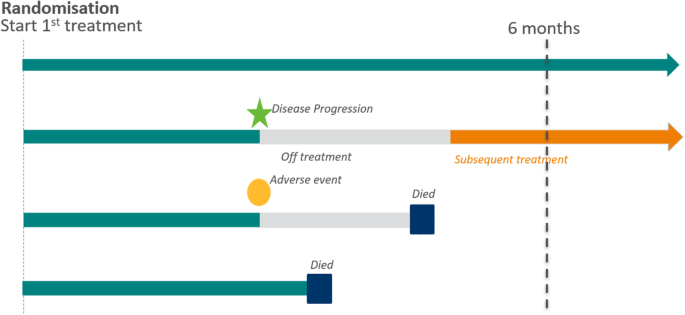
What is an estimand & how does it relate to quantifying the effect of treatment on patient-reported quality of life outcomes in clinical trials? | Journal of Patient-Reported Outcomes | Full Text

ICH E9 guideline 'Statistical principles for clinical trials': a case study Response to A. Phillips and V. Haudiquet - Brown - 2003 - Statistics in Medicine - Wiley Online Library

TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May PDF Free Download

PDF) ICH guideline “Statistical Principles for Clinical Trials”: issues in applying the guideline in practice