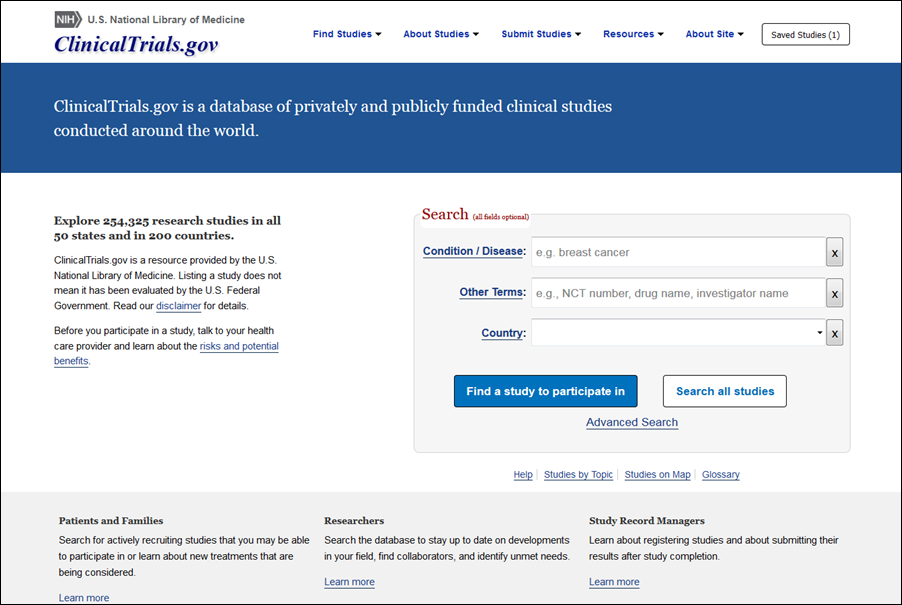
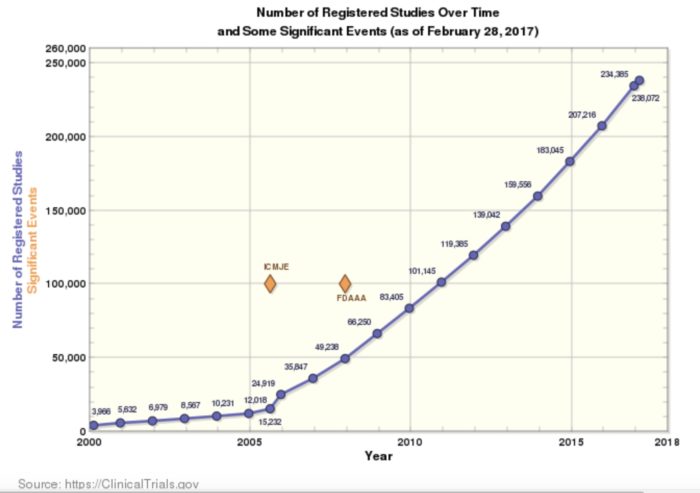
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

Post-Trial Responsibilities - The Multi-Regional Clinical Trials Center of Harvard and Brigham and Women's Hospital

Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine
PLOS ONE: Differences in Investigator-Initiated Trials between Japan and Other Countries: Analyses of Clinical Trials Sponsored by Academia and Government in the ClinicalTrials.gov Registry and in the Three Japanese Registries

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005 | The BMJ