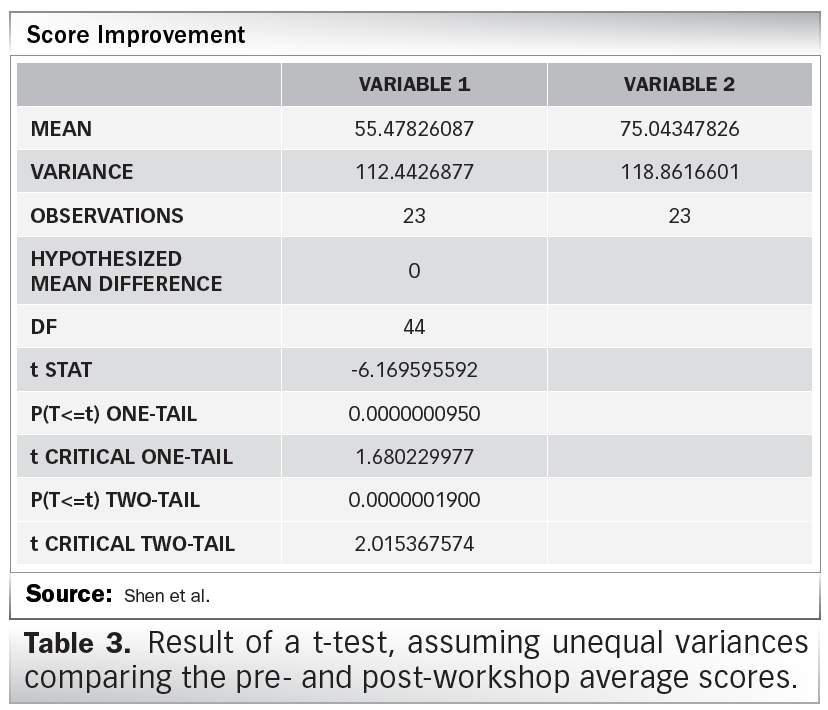
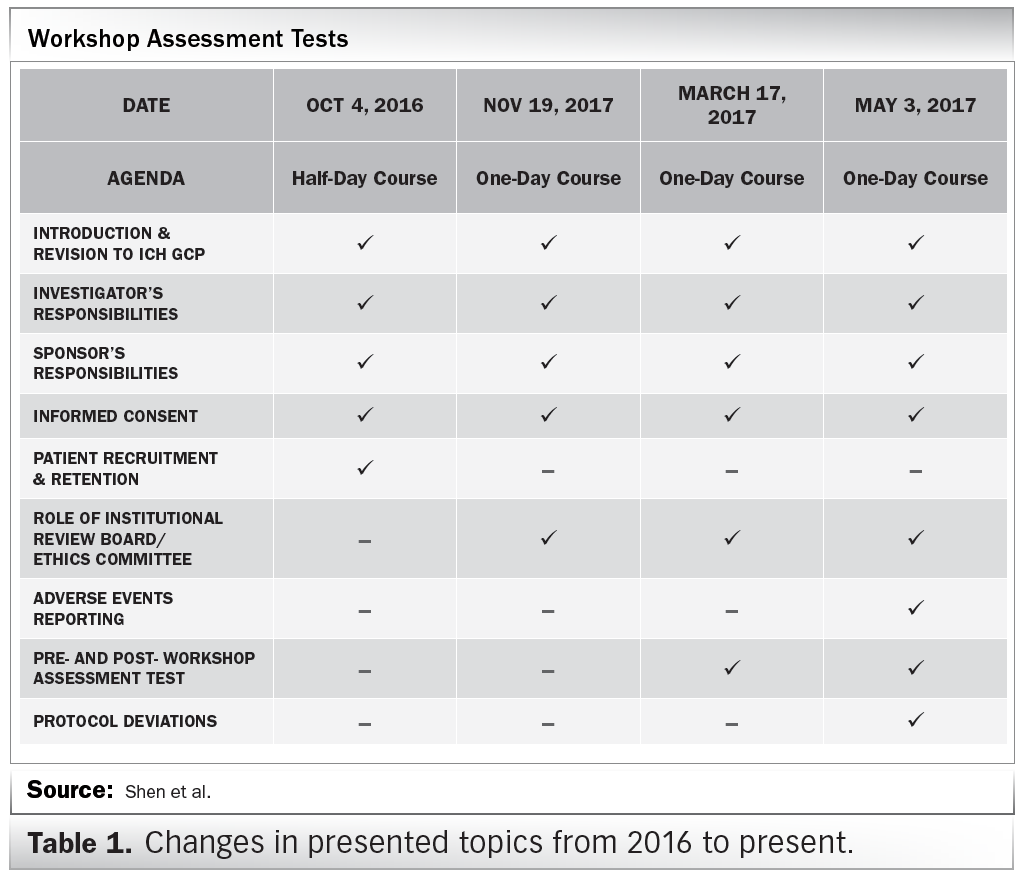
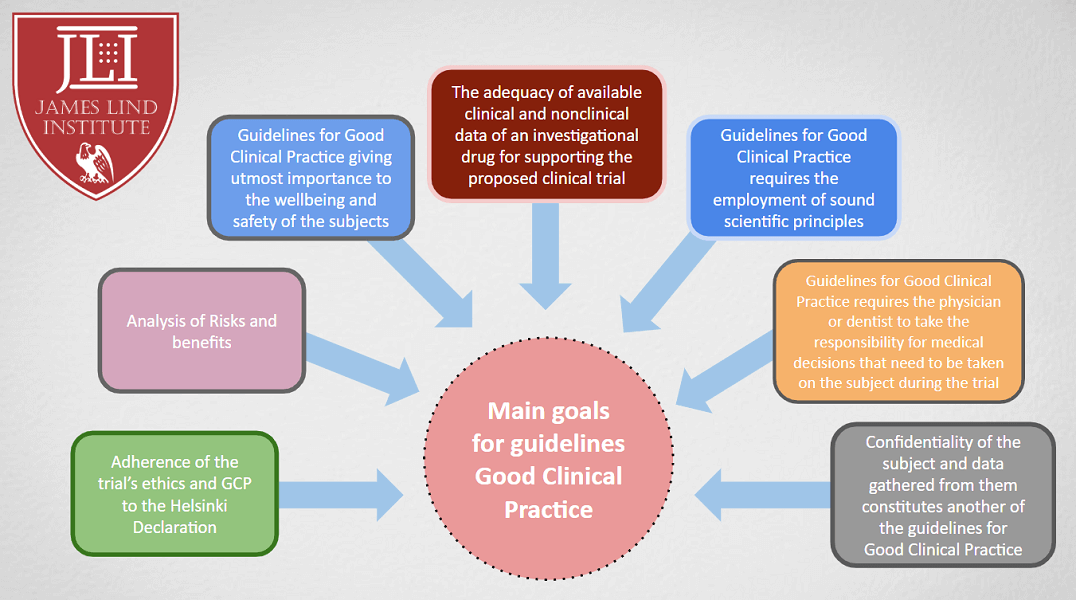
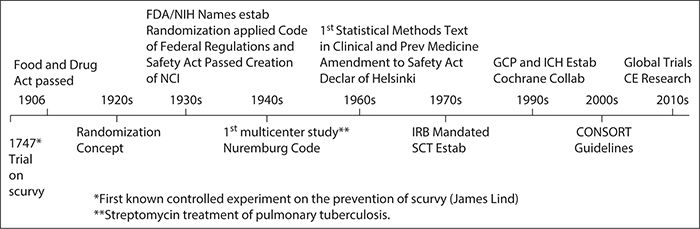
EU clinical research framework. ICH GCP = International Conference on... | Download Scientific Diagram

PPT - Good Clinical Research Practice Guidelines For Informed Consent PowerPoint Presentation - ID:5637042

TASK - COURSE ANNOUNCEMENT: TASK Academy is presenting a Good Clinical Practice Beginners course – including the latest edition to South African GCP (SA GCP third edition 2020). What is this course

PDF) Voluntary informed consent and good clinical practice for clinical research in South Africa: Ethical and legal perspectives

Book M1: 2021 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC

More than a box to check: Research sponsor and clinical investigator perspectives on making GCP training relevant - ScienceDirect